With the increasing improvement of people’s living standards and the importance of environmental protection, the new “green consumption” concept began to implant in people’s minds. At the same time also put the control of clothing fabric safety and environmental protection indicators of the subject in front of us. The safety and environmental protection indexes of textiles include many items. Among them, the determination of textile pH value is also updated with the concept of people’s consumption and gradually for the manufacturers and merchants to pay attention to. This article shows you how is pH tested and ensures how textiles’ pH value can be qualified.
What is the pH Value in Garments
The pH value in garments refers to the residual acid and alkali content in garment fabrics, which is determined by testing the pH value in the water extraction solution of fabrics, and is a technical indicator for controlling acidity and alkalinity in the production process. So the test for acidity and alkalinity of the garment is of great importance.
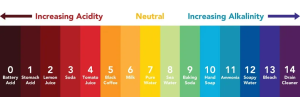
The determining pH value ranges from 0 to 14, pH equal to 7 is neutral, and pH less than 7 is acidic, the smaller the value, the stronger the acidity, pH more than 7 is alkaline, the larger the value, the stronger the alkalinity.
The Source of pH Value in the Fabric
The clothing production process in the chemical treatment process, such as fiber production, pulp dyeing, printing, washing, etc., are to be a solid color, and restore the cleaning (soaping). The process requires the use of a large number of soda ash, caustic soda, pH adjusters, surfactants, etc., coupled with fabric raw materials, organizational specifications processing equipment, and different production processes. Especially in the production process of water washing (neutralization) is not thorough enough, resulting in the existence of acid and alkali residues on the fabric.
Reasons for pH Exceeding
Influence of dyes in production
Commonly used reactive dyestuffs, serin dyestuffs, and sulfur dyestuffs for dyeing are produced under alkaline conditions, even if the cloth can be washed and treated well, it will be affected by the pH value of the water used for production.
Influence of printing and dyeing process
Cotton, wool, silk, polyester, nylon, acrylic, and other textiles after refining, dyeing, printing, and other processes, fabric residual alkali, acid chemicals, and additives, and there are different pH values. After washing, soaping, acid neutralization, drying, and other post-processing, if the amount of chemical additives is high or the post-washing treatment is not sufficient, the pH value of the textile will be exceeded, affecting the performance of the textile.
Influence of fabric
Different thickness of the fabric affects the pH value of the cloth surface, thin fabrics are easy to wash after dyeing, and the pH value of the cloth surface is low. Thick fabrics are relatively difficult to wash after dyeing, and the pH value of the cloth surface is relatively high.
Influence of operation error on laboratory personnel
Different dryness and humidity of the test fabrics, different extraction temperatures, different extraction times, etc. will affect the results of the pH value of the cloth.
The Danger of pH Value Exceeding
Normal human skin pH value is between 5.5 to 6.5, chemical reaction is weakly acidic, and the skin’s sweat glands and sebum secretion of sweat and oil itself has a pH. If the difference between the pH value of the outside world and that of the body’s normal needs is too great, it will cause people to feel unwell and many diseases will appear on the skin. Textile pH testing has been developed to meet this need. deviation of pH value from the standard value, resulting in inappropriate acidity or alkalinity of the garment, will lead to the destruction of the natural barrier of the skin surface. The body’s own neutral and acidic skin is irritated, producing a burning sensation, harboring bacteria, and causing illness.
pH Test Standards
(1) Chinese Standard (GB/T 7573-2002)
Weigh 3 portions of 2±0.05g specimens, cut them into small pieces of 5×5 mm, and put them into a flask containing 100ml of distilled water or a flask containing 100ml of potassium chloride solution (0.1mol/L). The flask was shaken to wet the specimen well. The flask was placed on an oscillator and shaken for 1h. The pH of the aqueous extract (without fabric) was determined directly at room temperature using a pH meter. Three tests were done for each sample, and the average of the results of the second and third specimens was taken as the final result, accurate to 0.05.
(2) European Standard (EN 1413-1998)
Weigh three portions of 2 ± 0.05 g of the specimen, cut them into 5 x 5 mm pieces and place them in a flask containing 100 ml of potassium chloride solution (0.1 mol/L) or 100 ml of distilled water. The flask was shaken to wet the specimen well. After placing the flask on a shaker for 2h, the pH of the aqueous extract (without fabric) was determined directly at room temperature using a pH meter. Three tests were made for each sample, and the average of the results of the second and third specimens was taken as the final result, accurate to 0.1.
(3) Japanese Standard (JIS L 1096-1999)
Weigh 5 ± 0.1 g of the specimen and cut it into small pieces of 10 x 10 mm. After boiling in 50ml distilled water and keeping it for 2min, put the specimen in a flask, shake the flask to make the specimen fully wet, and leave it at room temperature for 30min. then remove the specimen, and when the temperature of the aqueous extraction solution reaches 25±2℃, use a pH meter to directly determine the pH value of the aqueous extraction solution, accurate to 0.1.
(4) American Standard (AATCC 81-2001)
Weigh 10±0.1g of the specimen, cut it into small pieces boil it, and keep it in 250ml of distilled water for 10min. The specimen was placed in a flask and the flask was shaken so that the specimen was well moistened, The boiling was continued for 10 minutes and cooled to room temperature. The specimen was then removed and the pH of the aqueous extract was determined directly using a pH meter to an accuracy of 0.01.
pH Sampling Rules
The following rules apply to sampling for formaldehyde, pH, and anodyne dyes test
① Products with a color pattern, cut and mixed as a sample.
② Products with regular patterns, sampling by cycle, cut and mixed as a specimen.
③ The products with a large pattern cycle, sampling according to the proportion of the area of the ground and flower, cut and mixed as a sample.
④ For independent pattern products, the pattern area can meet a sample, and the pattern can be sampled separately; the pattern is very small less than a sample, the sampling should include the pattern, it is not appropriate to cut from multiple samples and then combined into a sample.
⑤ If the pattern is small or less than one sample, the sample should include the pattern and should not be cut from several samples and combined into one sample.
⑥ For products that can be manually layered, the samples shall be taken in layers and measured separately.
⑦ For products that cannot be manually layered, take the sample as a whole.
pH Sampling Device
The ProPress Sample Press cuts samples into small pieces (e.g. 5×5 mm) in seconds, automatically and with precisely controlled pressure applied to a precision die to cut the samples in a single pass and with precise dimensions for more reliable results in formaldehyde, pH, and more. The efficiency is increased by more than 80%, which is 6 times of manual cutting.
This sample cutter is suitable for textile fabrics, leather, plastics, and other flexible sheet material formaldehyde, pH, azo, heavy metals, and other test samples punching and cutting.
The testing principle of this sample fabric-cutting machine is that the samples to be punched are laid flat on a tray, the machine is started with both hands and the tray is pulled in. The ProPress automatically punches the samples and pushes them out when they are finished. This machine is equipped with a strong air cleaning system, which blows out all kinds of fibers and debris left behind by the punching and ensures that the samples are free of any mixing with each other.
Adjustment of Fabric pH Value
When the pH of fabrics or garments is unqualified, the pH of fabrics is usually adjusted by the principle of acid-base neutralization. pH unqualified is mostly alkaline, and many kinds of acids can be used to adjust it, the most common ones are glacial acetic acid and citric acid. Glacial acetic acid is volatile and has a pungent odor, while citric acid is more stable. Therefore, it is generally recommended to use citric acid to adjust the more appropriate, but pay attention to control the dosage, too much will affect the feel, and also easy to cause yellowing.
For example, if the fabric measured a pH value of 9 to 10, you can adjust the pH value of the citric acid solution for 4 to 5 and then soak it for 10 minutes, drying can be done. Before sending it to the customer, send it to the formal testing structure to confirm the qualification and reduce the trade risk.
Analysis of the Influencing Factors of Textile pH Value Test
From the blank fabric to the finished product, after pre-treatment, dyeing, and finishing these processes, the acidity and alkalinity of the fabric will have obvious changes. The reproducibility of data in the actual testing process of pH value is poor, especially in the critical value of the product, the sample needs to be retested to ensure the accuracy of the data. When determining the pH value of textiles, the following factors should be noted, and each test link should be strictly controlled.
(1) Different test standards and methods, the required test temperature, extraction time, and sample weight are not the same, resulting in very different results. Specific examples of standards in which pH measurement methods include: GB/T 7573-2009 “Textile-Determination of pH of aqueous extract”, ISO3071-2005 “Textiles – Determination of pH of aqueous extract”, AATCC 81-2016 “pH of the Water-Extract from Wet Processed Textiles”.
(2) The extraction temperature plays a decisive role in the pH test, with the increase of the extraction temperature, the pH test results show an upward trend. Therefore, we should pay attention to the temperature of the extraction solution when extracting, and also pay attention to the temperature of the testing environment when using the pH meter for testing.
(3) The pH meter should be calibrated and the accuracy of the meter should be observed because the glass electrode is easily broken or damaged, which may cause wrong results. Therefore, it is necessary not only to calibrate the pH meter with a buffer solution before the test but also to verify the instrument during the comparison.
(4) The calibration of buffer solution, generally takes 4.01, 6.86, and 9.18 three points as the calibration point, after calibration these three points must be ≥ 95% of the linearity, or need to recalibrate or replace the calibration buffer solution.
(5) The experimental water must meet the test requirements, the conductivity must be less than 0.50 mS/m, the pH value must be between 5.0 and 7.5, and cannot exceed the interval, otherwise it will affect the pH value of the sample itself.
(6) The different parts of the test sampling will have an impact on the test results. Containing printing, embroidery, and lining parts, obviously different from other parts of the results, especially after washing cotton and denim finished products, the sample itself is not uniform pH. In the same garment, the results of the front piece and sleeve may be different, and the front and back pieces of the trouser legs may be different. Therefore, it should be strictly assessed by taking the parts with folds, prints, and embroidery.
(7) The operation of the personnel will also produce differences, such as test personnel on the extraction of liquid shaking the degree of intensity will cause different results.
How to Ensure the Qualification of pH Value on Textiles
Dyeing and finishing process
The pH value of a textile is determined by each link of the dyeing and finishing process, from pretreatment to dyeing and printing as well as finishing and washing, all of which have an important influence on pH value. For example, a large amount of alkaline agent should be added to the refining of cotton in pre-treatment, acid should be added to the carbonization of wool, and when dyeing, wool, and silk are generally dyed in acidity, while cotton generally needs alkaline. After finishing need to add a large number of different acid and alkaline finishing agents and so on. Therefore, to get a satisfactory pH value for the finished extract, it is necessary to strictly control the pH value in each process of printing and dyeing process, and strengthen the washing process in each process of dyeing and finishing process.
Water washing is the occurrence of sufficient physical exchange and flushing thinning effect, is the dyes and a variety of chemical additives from the fibre pore solution diffusion, in the occurrence of desorption, by the water washing solution dilution exchange removal, so that the pH value on the fabric up to standard. Therefore, in addition to increasing the washing power of water washing on the surface of the fabric, also by increasing the water washing bath ratio, after washing with acid neutralization and adjusting of pH value, to improve and improve the pH value of the fabric.
Washing bath ratio
After many times of production, the standard deviation of the pH value of fabric after the dyeing and finishing process is gradually reduced with the increase of bath ratio, and the smaller the standard deviation is, the better the stability of the pH value is.
As a result of the printing and dyeing process, a large number of alkali molecules are attached to the fiber surface and fiber pores. Therefore, the larger the bath ratio is, the larger the concentration of alkali molecules on the surface of the fiber and in the orifices, the larger the difference between the concentration of alkali molecules in the washing liquid and the larger the energy; the faster the molecular movement spreads, and the easier the alkali dilutes and exchanges the wash away.
Acid neutralisation
After processing by dyeing and finishing treatment, there is still some alkali residue on the fabrics, especially the thick and tightly organized fabrics. As alkali molecules on the fiber have a certain affinity, washing is not easy to diffuse out of the fiber pores. There are also mercerized fabrics, due to the high alkali concentration (sodium hydroxide in 245-300g/l), it is difficult to remove alkali molecules when washing. Therefore, to control the pH value of the fabric, it can be neutralized with 0.3-0.5g/L glacial acetic acid and 0.1-0.25g/L sodium acetate buffer solution.